Did you know?
You can double click on a word to look it up on TermGallery.
You can double click on a word to look it up on TermGallery.
Meanings of polar molecule in inglés
ruso
полярные вещества español
molecula polar 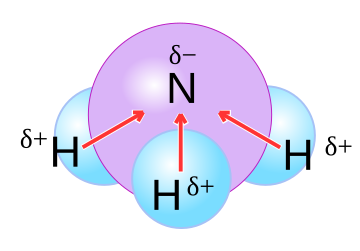
Molecule, interact through dipole–dipole intermolecular forces and hydrogen bonds, must contain polar bonds due to a difference in electronegativity between the bonded atoms.
See moreUsage of polar molecule in inglés
1
That's because water is a polar molecule, while grease or oil is a nonpolar molecule.
2
Water does this because it is a polar molecule.
3
Such an asymmetric bilayer is a highly effective barrier for polar molecules.
4
Polar molecules like water attract each other like magnets, too.
5
The polar molecules again try to orient themselves in sympathy with the fast undulations of the field.
6
A hydrogen bond results from the attraction between oppositely charged ends of polar molecules (or portions of molecules).
7
Multiple assays are used to show that FliP suffices to form a channel that can conduct a variety of medium-sized, polar molecules.
8
Temperature-dependent molecular desorptions for six different polar molecules were measured in real-time to study the desorption kinetics and extract the binding affinities.
9
The water molecules are polar and form strong intermolecular bonds with other polar molecules, which are able to balance out their electrical charges.
10
Furthermore, the heterodyne sensing technique enables electrical probing and tuning of the noncovalent physisorption of polar molecules on graphene surface for the first time.
This collocation consists of:
Translations for polar molecule
español

